FDA Authorizes Updated Covid-19 Vaccines for Vulnerable Populations
Opinion | 8/30/2025
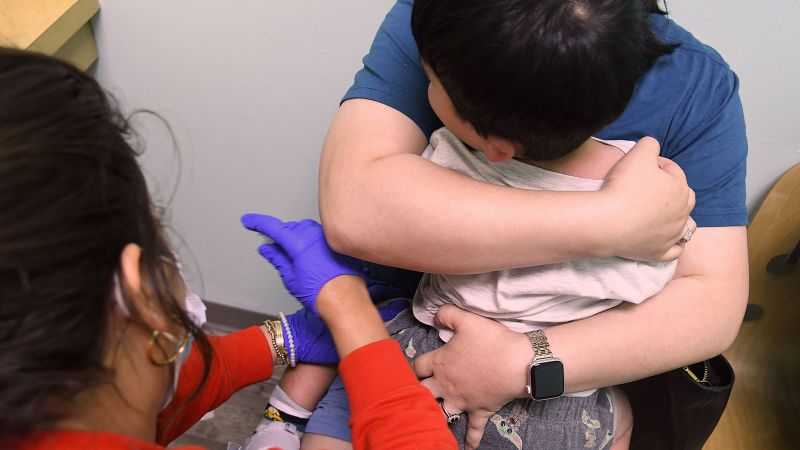
The US Food and Drug Administration has recently authorized updated Covid-19 vaccines for specific demographics. Individuals aged 65 and above, as well as younger individuals with certain medical conditions predisposing them to severe Covid-19 infections, are eligible for these updated vaccines. The move aims to enhance protection against the virus among vulnerable populations.
These updated Covid-19 vaccines come as an essential development in the ongoing efforts to combat the pandemic. By targeting older adults and individuals with specific medical vulnerabilities, health authorities seek to bolster immunity levels within groups at higher risk of severe illness or complications from the virus.
A White House official noted the importance of extending vaccine eligibility to these groups, emphasizing the need to prioritize their safety and well-being in the face of evolving pandemic challenges. The authorization underscores the government’s commitment to tailoring vaccination strategies to address the varying needs of different segments of the population.
Legal and ethical considerations have shaped the decision to expand vaccine eligibility criteria, reflecting a data-driven approach to public health policy. The FDA’s approval of updated vaccines for at-risk demographics aligns with broader efforts to mitigate the impact of Covid-19 on vulnerable communities.
As the vaccination campaign continues, health authorities stress the significance of ensuring equitable access to vaccines for all eligible individuals. By targeting specific age groups and medical conditions, the updated vaccine guidelines aim to optimize protection against Covid-19 within populations most susceptible to severe outcomes.